Stem cells offer tremendous promise.
Some clinicians have put the cart before the horse, selling the treatments after making bold claims. There’s a lot of money to be made servicing the demand. Here, the Federal Trade Commission and Georgia Attorney General said enough is enough.
This is how the case was captioned. CIVIL ACTION NO. 1:21-cv-3329-AT There were lots of defendants.
FEDERAL TRADE COMMISSION and STATE OF GEORGIA, Plaintiffs, v. STEVEN D. PEYROUX, individually and as an owner and officer of REGENERATIVE MEDICINE INSTITUTE OF AMERICA, LLC, also d/b/a Stem Cell Institute of America, LLC, PHYSICIANS BUSINESS SOLUTIONS, LLC, and SUPERIOR HEALTHCARE, LLC, BRENT J. DETELICH, individually and as an officer of REGENERATIVE MEDICINE INSTITUTE OF AMERICA, LLC, also d/b/a Stem Cell Institute of America, LLC, REGENERATIVE MEDICINE INSTITUTE OF AMERICA, LLC, a limited liability company, also d/b/a Stem Cell Institute of America, LLC, PHYSICIANS BUSINESS SOLUTIONS, LLC, a limited liability company, and SUPERIOR HEALTHCARE, LLC, a limited liability company, Defendants.
By decision rendered January 8, 2024, federal court ordered the assorted defendants to pay $5,155,146 in both civil penalties and refunds to defrauded consumers on Georgia’s state law claims.
Some background:
The agencies’ 2021 complaint named Steven D. Peyroux and Brent J. Detelich; Regenerative Medicine Institute of America, LLC, doing business as Stem Cell Institute of America, LLC (SCIA); Physicians Business Solutions, LLC (PBS); and Superior Healthcare, LLC (SHC) as defendants.
In 2015, Peyroux, a chiropractor, and Detelich, a former chiropractor, co-founded SCIA, a company that trained chiropractors and other healthcare practitioners how to deceptively market unproven stem cell therapy in their practices. SCIA trained its client clinics how to recruit patients through advertising, host free “educational seminars,” and conduct consultations. SCIA provided its clients access to a “vault” of sample advertisements rife with baseless claims of efficacy, and the appearance of being part of a nationwide SCIA network.
The defendants also used these deceptive marketing materials and “educational seminars” to attract stem cell patients to their own chiropractic clinic, SHC. SHC charged up to $5,000 per stem cell therapy injection, with many patients receiving more than one injection as part of their treatment. The group of consumers who purchased defendants’ unproven stem cell therapy consisted almost exclusively of elderly and disabled people.
In terms of the cast of characters:
Peyroux, a licensed chiropractor (PSOMF-Peyroux, Doc. 115-1 ¶ 134), was the 100% owner of all three Corporate Defendants: Superior, Physicians Business, and SCIA. (Id. ¶¶ 138, 156, 184.) Peyroux was the Executive Director and a corporate officer of Superior. (Id. ¶¶ 185–86.) Peyroux is the CEO and a corporate officer of Physicians Business. (Id. ¶¶ 157–58.) Peyroux was a co-founder and corporate officer of SCIA. (Id. ¶¶ 136, 139.)
Detelich was licensed as a chiropractor, but his license was revoked after he was convicted in federal court of several felonies and because he committed unprofessional conduct. (PSOMF-Detelich, Doc. 117-1 ¶ 245.)4 Detelich was a cofounder, officer, and director of SCIA and served as SCIA’s president until Peyroux took over this role in January 2018. (Id. ¶¶ 249–52.) As to Physicians Business, Detelich was directly involved in developing, marketing, and delivering consulting services about stem cell therapy. (Id. ¶ 304.) Detelich was also directly involved with Superior’s marketing of its stem cell therapy treatments (though Detelich claims that he was involved only because Superior was a client clinic of SCIA). (Id. ¶¶ 314–27.)
In 2019, Superior and SCIA filed for bankruptcy. (Id. ¶¶ 3, 8.) The bankruptcies closed in August and November 2021. (Id.)5 Physicians Business remains operational. Peyroux and Detelich currently hold interests in several other healthcare companies. (PSOMF-Peyroux, Doc. 115-1 ¶¶ 217, 218, 219, 229, 230, 234, 238, 241; PSOMF-Detelich, Doc. 117-1 ¶¶ 332, 334.)
The FTC and Georgia AG argued that stem cell advertisements were deceptive. They misrepresented the efficacy of stem cell therapy treatment. There were many examples. See highlights below:
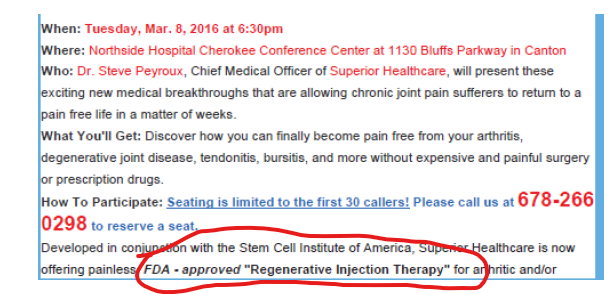
Superior also used TV ads. (See Superior TV Ad Video, Doc. 78-6, Pls. Ex. 131, filed manually) (“It only takes one stem cell treatment to reduce or eliminate your knee pain without prescription medications and without surgery. In fact, many patients start experiencing relief from their pain immediately after the treatment.”); (See also Superior TV Ad II, Doc. 95-24, Pls. Ex. 574, filed manually) (advertising stem cell therapy as treatment for neuropathy).
For example, on its website, Superior stated, among other things, “OUR . . . STEM CELL TREATMENTS CAN PROVIDE REMARKABLE IMPROVEMENT WHEN OTHER TRADITIONAL MEDICAL PROCESSES HAVE FAILED OR HAD LIMITED EFFICACY.” (See Superior Website, Docs. 96-24, 126-2 at ECF 21) (also noting “stem cells can be used to treat nearly any type of condition caused by injury or degeneration”). Superior’s website also advertised for “educational seminars,” by offering attendees a chance to learn about this “cutting edge therapy” that could provide relief in “as little as one treatment”
Defendants also served as marketers.
Beyond advertising directly to consumers, SCIA and Physicians Business — as consultants — provided ads to client clinics for those clinics to use when advertising stem cell treatments and lectures…
One sample magazine/newspaper ad touted the stem cell therapy as “FDA-approved,” as shown below:
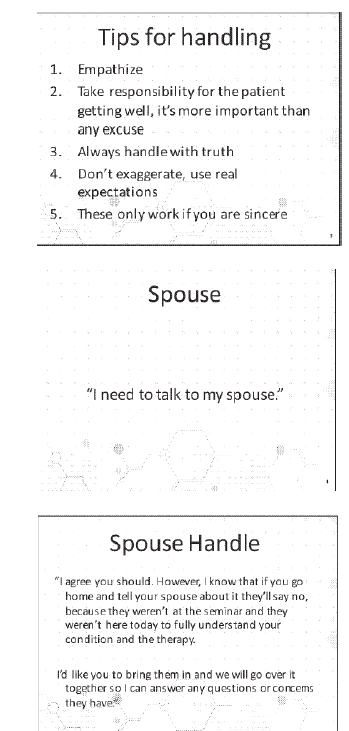
The marketing course identified tips for handling objections, such as “I need to talk to my spouse”:
Below is a script for “handling objections” in a training video:
There are certainly some research and things like that that I can provide to you that are published studies in well-known journal articles. However, the question you really should be asking me is . . . how many people and what results have you seen in people with similar knee conditions to me. . . .
Over the past few years that we’ve done this, we have treated hundreds of patients with knee conditions with amazing results. So the level of degeneration you have in your knee makes you a really good candidate for this procedure. And I feel very confident in telling you that this is going to be a positive outcome for you as far as improving your range of motion, quality of life, and decreasing pain.
(Objection Training Video, Pls. Ex. 470, filed manually.)
The pitch to train clinicians:
Physicians Business also hosted seminars at the client clinics themselves that were directed to the clinics and/or the public. Such seminars could include sessions titled “Cash Services that Bring in New Patients by the ‘Truckload’” and “Miracles of Stem Cells and Your Office,” (Garden State Seminar Ad, Doc. 75-20 at ECF 9), or “How to resolve a knee condition with certainty in just 1-2 treatments – The secret to getting $5,000-$10,000 case fees paid in cash for these conditions” (Apex Seminar Agenda, Doc. 90-5). At one Physicians Business Seminar in 2021, the agenda included talks by “Dr. Brent [Detelich]” and “Dr. Steve [Peyroux]” about these topics, among others: “Learn the only FDA approved regen[erative] med[icine] systems that get results!” (emphases added); “How to stay compliant with FDA and FTC for treatments and marketing”; “Best systems to ensure 5 to 10 K per case”; “Cellular Hope Institute – Your private personal advanced Regen[erative] Med[icine] treatment Hospital in Cancun, Mexico . . . . Learn our ultra smooth process of preferring patients to the cancun[sic] center [and] Legal documents so that you can receive 20% of all funds collected without any risk.” (Marietta Seminar Agenda, Doc. 77-25.) Physicians Business also offered Facebook Live events about stem cell therapy and exosomes.8 (See PBS Facebook Live, Doc. 92-15.) In advertising for virtual sales trainings, Physicians Business represented that its regenerative medicine programs were both “FDA approved” and “approved by the FTC & FDA,”…
Well, what happened?
On December 26, 2024, the court issued orders for injunctive and monetary relief. The first order permanently bans the defendants from advertising, marketing, promoting, offering for sale, or selling any regenerative medicine treatments, including any treatment or therapy that falls under the definition of stem cell therapy.
It also prohibits them from misrepresenting that any regenerative medicine compliance training program is approved by either the FTC or the U.S. Food and Drug Administration. Finally, it prohibits the defendants from providing others with the means of making false and misleading statements about regenerative medical treatment.
The second order, based on Georgia’s state law claims, requires Peyroux and Detelich to pay $3,310,146 that may be used to provide refunds to defrauded consumers, and Peyroux, Detelich, and PBS to pay $1,845,000 in civil penalties, resulting in a total monetary penalty of $5,155,146.
Interestingly, in other stem cells legal cases, the providers made bold claims about hard-to-treat conditions, such as Parkinson’s, ALS, MS, etc. Here, the claims were for managing plain vanilla chronic pain and joint immobility.
In addition, the defendants overstepped advertising the therapies were FDA-approved.
Right now, in office stem cell treatments by practices are not FDA-approved. There are likely several clinical trials ongoing as I write. If they succeed, they may be on the road to later FDA-approval.
It’s possible that Congress / FDA will carve out new laws / regulations for stem cells. But for the moment, the cases are being adjudicated by laws on the books.
Tread carefully.
What do you think?






Well, were they actually using stem cells? If so, whose? How did they obtain them? If they were using the patients’ own cells, how did they get them and purify them? Seems unlikely that a couple of chiropractors would have the laboratory expertise necessary to extract a patient’s own cells and either regress them to stem cells or locate them–unless, of course, they somehow magically had access to a patient’s stored frozen umbilical cord for them. But if the patients are Boomer generation, they won’t, of course, have such access since that wasn’t being done back then.
So…details are lacking here. Please provide.
And…if they weren’t injecting stem cells, what ~were~ they injecting? At that point, there could not have been informed consent, which means that it was at least assault and likely battery. How many patients did they dupe?
This is just another multi level marketing system.
Interesting that so many of these occur from chiropractors, BECAUSE chiropractors get paid so little for doing adjustments that they all need to figure out some way to make a living.
Chiropractors that I know of, quit taking insurance altogether because of the terrible reimbursement 30 years into their practice.
Even among physicians so many scandals seem to occur because of limited income potential just practicing straight medicine, without performing procedures.
IF one takes out the financial benefit, most of these scams would not be successful because the chiros or docs would not be tempted. They are temped because they are not making it financially.
What is truly ironic here is that stem cells are used successfully throughout the rest of the developed world for treatment of various diseases; and osteoarthritis is one of the most common and most successful uses of stem cells. Yet our FDA (with its takeover by big pharma) declared, following an inappropriate use and administration in they eye of a patient in FL, that “stem cells are ‘unproven’ and dangerous”. So here, FTC and the state of Georgia pounced upon and ultimately ruled these individuals were using “false and deceptive advertising” to promote “unproven and dangerous therapies.”
It seems to me that their biggest mistake was implying that any such treatment is FDA approved. Oh, yes, and the aggressive marketing scheme was probably offensive on its face to anyone who is already prejudiced against the entire field of stem cell therapy. And as comments suggest on this forum, also offensive to any physician who believes (as I do actually) that chiropractors (especially a defrocked one) should be “adjusting spines” (or whatever it is that they actually are trained to do) and not practicing medicine.
Sadly, the injunction was levied against any future use of the term “Regenerative Medicine”, a field that includes dextrose and PRP prolotherapy as well as shockwave therapy, which treatments are both highly effective and do NOT administer stem cells, but rather recruit the patient’s own from elsewhere in the body to the site of debility.
The injunction only affects these providers, but let’s hope other courts don’t follow suit and throw the baby out with the bathwater. Meanwhile, if you truly continue to believe that steroids in joints (or even nonsteroidals for joint pain) will improve your osteoarthritis, I know of a great bridge in Brooklyn on which I could get you a great price. Both of these agents are paths to an orthopedic surgeon’s preferred mode of treatment.
We’re seeing a lot of this lately. To me, it’s the federal law enforcement agencies self-funding and in love with asset seizure and lawsuits. Healthcare professionals are not safe anymore…it’s apparently bad to make money, so they take it. This could very well be a result of the destruction of pain management, with over 4,000 providers (and counting) being charged with false allegations of “overprescribing”. While there was liberal prescribing in the 90s and early-mid 2000s, it was encouraged by the government.
They lied about the “opioid crisis” and hid the fact that they knew it was illicit fentanyl driven. This was a litigation narrative that many joined…and now it has led to disastrous results. There are desperate people seeking relief via the illicit market, including the elderly and disabled. Big Pharma wants all physicians/clinicians to only use Suboxone for pain-which is totally inappropriate and comes with its own risks, including increased overdose risk-they buried a 2012 study that showed this (mu receptor “overload”). This is also concerning in the realm of surgery. A patient in need of anesthesia has to come off of Suboxone before receiving anesthesia. The withdrawal, as described by heroin/illicit drug users, is harder than heroin itself.
We need to return to a fair and balanced approach to pain management. The people who claim to have had surgery and suddenly became iv drug users aren’t telling the whole story-they likely (99%) had a substance abuse problem beforehand but this is an easier way to admit it, as it’s shameful and difficult to admit to…blaming a doctor is much easier).
(Heroin withdrawal *)
“…osteoarthritis is one of the most common and most successful uses of stem cells.”
This is a strong statement and seems quite different than my understanding of the state of the art in treating osteoarthritis. Having lectured a grand rounds on this topic and suffered for decades before knee replacement I would have stated that exercise (with caveats) and weight loss are the known ways to treat the pain and disability, true disease modifying therapies have been theorized and tried with failure for over 50 years.
I find it fascinating that there are regenerative medicine departments in the tertiary and quaternary centers (one of which I have close contact) offer only a couple of types of therapy but out in the community stem cells “work” for every complaint.